The New York Times
A bill approved by the Senate yesterday to spur stem cell research would go a long way toward removing restrictions that have slowed progress, burdened laboratories with red tape, reduced American competitiveness and discouraged young researchers from entering the field, several leading stem cell scientists said.
Still, some of the scientists said, even if the legislation escaped a promised veto by President Bush, it would not remove all restrictions on federal financing of their work. And the legislation by itself would not immediately provide an overall increase in money for the research, just allow researchers to spend the money they have more broadly.
”It would make a major impact, but there wouldn’t likely be a windfall of funding in this area,” said Dr. Arnold Kriegstein, director of the Institute for Regeneration Medicine at the University of California, San Francisco. Some researchers hope the legislation would lead eventually to more financing, though the federal research budget has been tight.
The legislation would end a policy put in place by Mr. Bush that restricts federal financing for human embryonic stem cell research only to cell lines, or colonies, that were derived on or before Aug. 9, 2001, the day the policy was announced.
That would free scientists to use federal money to do experiments with many of the scores of stem cell lines that have been derived since then either in other countries or with private money in the United States. Federal financing would still be restricted to stem cell lines derived from embryos that were slated to be discarded by in vitro fertilization clinics.
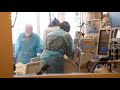
Embryonic stem cells have the potential to turn into virtually any type of body cell. Scientists hope one day to produce tissues to repair the damage caused by Parkinson’s disease, diabetes and other diseases.
But creating the stem cells now involves the destruction of human embryos, which some people, including Mr. Bush, say is immoral. Supporters of the new bill do not appear to have enough votes to override a presidential veto, so the existing restrictions are likely to remain.
Scientists say those restrictions have been a big hindrance. There have turned out to be only about 20 cell lines that qualify for federal financing, not more than 60 as the government initially said in 2001.
Those older cell lines, because they were grown using animal cells or serum, might not be suitable for use as medical therapy. And over time some of the cells have accumulated genetic abnormalities that make them more difficult to use even for basic research.
”They are behaving bizarrely,” Dr. George Q. Daley, associate professor at Harvard and Children’s Hospital Boston, said of one of the cell lines his laboratory had been nurturing for five years.
Scientists have been able to derive and work with newer cell lines, but only by using private or state money. They have to segregate the work using the new cells from the federally financed work, causing duplication and red tape.
The University of California, San Francisco, for instance, is spending more than $5 million to set up a laboratory on campus, duplicating much of the equipment already available in other laboratories, Dr. Kriegstein said.
Even under the new legislation, scientists said, they could still not use federal money to create new cell lines if it involved the destruction of embryos. Nor could federal money be used for therapeutic cloning, in which stem cells are created from a body cell of a person.
Such therapeutic cloning might be used to create stem cells from people with specific diseases. It might also allow replacement tissues to be made that are genetically matched to a patient, so they would not be rejected when implanted. Both Harvard and California, San Francisco, have announced plans to do therapeutic cloning using private money.
Still, it is difficult to quantify how much the president’s policy has actually retarded research. Private donations worth tens of millions of dollars have filled the gap to some extent, though scientists say the federal government would be a larger and steadier source of money. A few states are also putting money into the field, the biggest by far being California, which is slated to spend $3 billion over 10 years, though the money is now held up by litigation.
”Scientists are tenacious and resourceful, so we figure out ways to get our work done regardless,” said Dr. Evan Snyder, professor and director of the stem cell program at the Burnham Institute for Medical Research in San Diego.
Dr. Snyder said the field had progressed ”exceptionally quickly,” though, he added, ”We’d be at warp speed if we had federal funding.”
Most scientists say it will be years before therapies from embryonic stem cells will be widely used, though some clinical trials could start within a few years. Some therapies derived from adult stem cells are already in use.
James Sherley, an associate professor of biological engineering at the Massachusetts Institute of Technology who objects to embryonic cell use on moral grounds, said adult stem cells ”can do everything we need” to provide therapies. But many other scientists say there are problems in isolating and multiplying adult stem cells.
With the field still in an early stage, much can be learned about how stem cells behave using the approved cell lines or by working with animal stem cells or human adult stem cells, which are not subject to spending restrictions.
The National Institutes of Health, while spending only $38 million this fiscal year for human embryonic stem cell research, is spending $571 million on other forms of stem cell research.
”It’s not that this has just been five years of downtime,” said Dr. Steven Goldman, a professor of neurology at the University of Rochester. ”Things have moved very quickly on parallel fronts.”
Still, scientists say that as the research now moves closer toward deriving treatments, it will be important to be able to use newer cell lines, like those from people or embryos with specific diseases.
”The presidential lines are generic cell lines and they allow you to ask very generic questions,” Dr. Daley said. ”The field has moved incredibly rapidly. We want to ask questions of real medical relevance.”
There is evidence that the existing policies have led to a loss of American competitiveness in the field.
A couple of studies have suggested that the United States accounts for a smaller proportion of the world’s scientific papers on human embryonic stem cells than on other fields of biological research, or a smaller proportion in 2004 than in 2002.
The research has been ”a little more robust outside the United States,” said Dr. Robert Goldstein, chief scientific officer of the Juvenile Diabetes Research Foundation International.
Still, some other factors could also be at work. Some scientists have said that research has been hindered somewhat by fundamental patents on human embryonic stem cells held by a foundation affiliated with the University of Wisconsin, where human embryonic stem cells were first isolated. Those patents are not in effect outside the United States.
Yesterday the Foundation for Taxpayer and Consumer Rights, a watchdog group in California, filed a request to the United States Patent and Trademark Office to invalidate the Wisconsin patents. The group, assisted by the Public Patent Foundation, said earlier work by other scientists had made the work done in Wisconsin obvious and not patentable.
The Wisconsin Alumni Research Foundation said it was confident that its patents were valid. It said that the patents did not inhibit research and that it had provided a free license and cells to 324 research groups.